Magnesium in the cell membrane regulates concentrations of other minerals
Some of the proteins that incorporate magnesium into their structure dot the surface of our cell membranes, performing a variety of roles like receiving signals from hormones (signal transduction), enzymatic activity, and transporting things across the membrane. In particular, magnesium-dependent proteins are used to facilitate the transport of different minerals into and out of cells, acting as gates for sodium (Na+), potassium (K+) and calcium (Ca+).
Many of these are active transporters, for instance pumping sodium out of cells even though it’s against the concentration gradient.
Think of a flooding basement. It’s raining and water naturally flows downhill. That’s why it is leaking into the basement through cracks in the wall.
But if you have a water pump, you will be able to pump that water back out of the basement, against the gradient of gravity.
That’s why we see much greater concentrations of sodium in the extracellular environment compared to the concentrations inside cells (and vice-versa for potassium). Magnesium-powered ion pumps maintain those specific concentrations. Ionic gradients serve various purposes in the body and cells. Different cellular actions are governed through varying the concentrations of these minerals.
Sodium and potassium conduct nerve signals
Sodium and potassium gradients are key to how nerve cells transmit electrical signals. When a cell receives a stimulus, the cell opens gates that allow sodium ions to rush into the cells and potassium ions to rush out. This action in one part of the cell membrane will cause nearby parts of the cell membrane to act as well, creating a travelling wave of depolarization. This wave is called the nerve impulse.
Without enough magnesium, the active transporters are unable to restore the original concentrations of sodium and potassium in the cell. This, along with a few other functions magnesium plays, can lead to an overactive nervous system which is more sensitive to random stimuli. In real life, that might translate to increased sensitivity to noise, irritability, migraines, twitching, irregular heartbeats and anxiety.
If left unchecked, a magnesium deficiency can also lead to a potassium deficiency, as potassium is released into the bloodstream and flushed out in urine.
Magnesium regulates calcium in cells
Calcium is used in cells as a cofactor for a variety of energetic functions including nerve impulses (like sodium and potassium), cell movement and most notably muscle contractions. Because calcium is typically an excitatory cofactor, the mineral usually enters a cell only when needed for something specific, like a nervous impulse or a muscle contraction. After such an action occurs, magnesium helps active transporters pump calcium out of a cell.
Like with sodium and potassium pumps, insufficient magnesium may prevent calcium pumps from working. Unable to flush out calcium, the cell may become overstimulated, damaging the cells and even leading to cell death (apoptosis). Over excitation in nerve or muscle cells might manifest as muscle spasms or twitching, and over time can cause neurodegenerative diseases.
The next time you are doing a high-intensity workout at the gym, see if you experience any muscle cramps. Those might be an acute sign your muscle cells not being able to restore calcium balance. Take some magnesium and see what happens. Muscle contraction is the classic example of how magnesium and calcium balance each other in the body and is the most easily observable.
And it’s not just contractions in your biceps. Magnesium-regulating calcium will affect the strength of heart and arteriole contractions too. Tension headaches are caused by too much muscle tension or contraction in the head and neck.
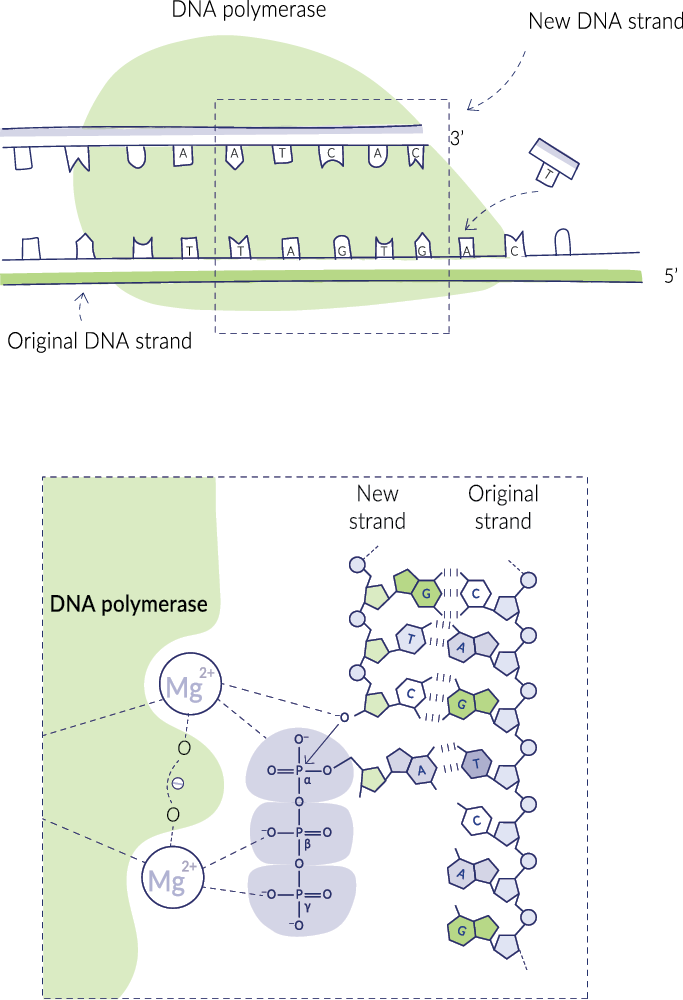
DNA polymerase is used all the time in DNA repair and DNA copying, creating new strands at a speedy rate of 3,000 nucleotides per minute. Consider the magnitude of the role this enzyme plays. Hundreds of billions of cell divisions occur in the body daily, and each time a cell divides, it needs to replicate an identical set of DNA, or approximately 3 billion base pairs.
DNA polymerase has two binding sites for magnesium. Without magnesium, it cannot work. This is corroborated by studies that show DNA synthesis visibly slowing in the absence of enough magnesium.
Repairing DNA
DNA polymerase is a very accurate enzyme, making less than one mistake in a billion base pairs. But even if DNA is copied perfectly, mistakes in the DNA sequences do occur. Genetic damage can occur because of thermal changes, radiation, viruses or the presence of highly reactive chemicals. There’s a lot that can go wrong when you’re maintaining 3 billion base pairs. If left unchecked, these mutations will be propagated with every cell division.
There is a whole other set of processes dedicated to identifying and correcting damaged DNA. The involved enzymes cut away the damaged sections and repair the gap with fresh nucleotides. Unsurprisingly, magnesium is involved in almost every enzyme in this process.
Protecting nucleotide bindings and proteins
Magnesium also has a stability effect on the structures of proteins and DNA. You might remember that for electrical charge, opposites attract and likes repel. No? Try rubbing two balloons against your hair. Now put them side by side. Because you’ve given them the same electrostatic charge, they will push apart. DNA is like that too.
Each strand in a DNA double-helix is negatively charged. Without the hydrogen bonds of their nucleotide base pairs holding them together, they will repel and break apart. In situations where DNA is exposed to higher temperatures or extreme pH, these hydrogen bonds can break. Magnesium ions have a strong positive charge.
Concentrated in the nucleus of cells, these ions can help reduce the negative charges in the DNA strands, stabilizing their structure.
This effect has been tested experimentally – a higher concentration of magnesium will measurably raise the melting temperature of DNA molecules.Many proteins and protein complexes incorporate magnesium into their structure – about 3751 human proteins with magnesium binding sites to date.





